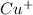Answer:
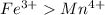 >
>
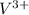 =
=
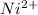 >
>
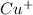
Step-by-step explanation:
Electronic configuration represents the total number of electrons that a neutral element contains. We add all the superscripts to know the number of electrons in an atom.
Fe: 26:
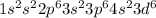
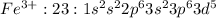 : 5 unpaired electrons
: 5 unpaired electrons
Mn: 25:
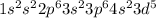
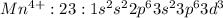 : 3 unpaired electrons
: 3 unpaired electrons
V: 23:
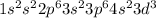
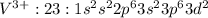 : 2 unpaired electrons
: 2 unpaired electrons
Ni : 28 :
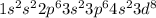
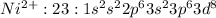 : 2 unpiared electrons
: 2 unpiared electrons
Cu : 29 :
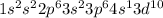
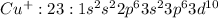 : 0 unpaired electrons
: 0 unpaired electrons
Thus the order of decreasing number of unpaired electrons:
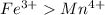 >
>
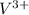 =
=
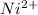 >
>