Answer:
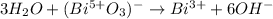
Step-by-step explanation:
Hello there!
In this case, according to the required half-reaction, we start by setting it up from bismuth (V) oxide ion to bismuth (III) ion:
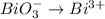
Thus, next realize that the oxidation state of Bi in BiO3^- is 5+ because oxygen is 2- (-2*3+x=-1;x=-1+6;x=+5), so we obtain:
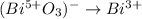
Thereafter, we realize three water molecules are needed on the right in order to balance the oxygens and consequently 6 hydrogen atoms on the left to balance hydrogen:
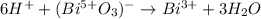
Now, since the balance is is basic media, we add six molecules of hydroxide ions in order to produce water with the hydrogen ones:
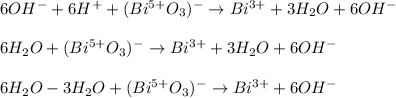
Then, we accommodate the waters to obtain:
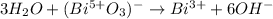
Best regards!