Answer: The tree died 9709.46 years before.
Step-by-step explanation:
Expression for rate law for first order kinetics is given by:
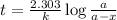
where,
k = rate constant
t = age of sample
a = let initial amount of the reactant = 100
a - x = amount left after decay process =
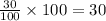
a) to find rate constant
Half life is the amount of time taken by a radioactive material to decay to half of its original value.
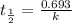
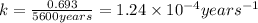
b) to know the age
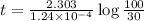
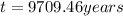
The tree died 9709.46 years before.