Answer:
a) λ = 4.862 10⁻⁷ m, b) λ = 4.341 10⁻⁷ m
Step-by-step explanation:
The spectrum of hydrogen can be described by the expression
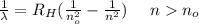
in the case of the initial state n = 2 this series is the Balmer series
a) Find the wavelength for n = 4
let's calculate
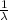 = 1,097 10⁷ (
= 1,097 10⁷ (
 )
)
\frac{1}{ \lambda} = 1.097 10⁷ 0.1875 = 0.2056 10⁷
λ = 4.862 10⁻⁷ m
b) n = 5
\frac{1}{ \lambda} = 1,097 10⁷ (
 )
)
\frac{1}{ \lambda} = 1.097 10⁷ 0.21 = 0.23037 10⁷
λ = 4.341 10⁻⁷ m