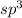Answer:The electron-domain geometry around this central atom is linear
Step-by-step explanation:
The hybridization is sp which means the number of electron pairs involved are 2. The electron domain geometry will have two sigma bonds and thus geometry will be linear.
The electron domain geometry will be Trigonal bipyramidal when hybridization is
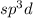
The electron domain geometry will be octahedral when hybridization is
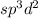
The electron domain geometry will be Trigonal planar when hybridization is
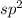
The electron domain geometry will be tetrahedral when hybridization is