Answer: The pH of the substance is 8.25. The solution is basic.
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydronium ion concentration. The acids have pH ranging from 1 to 6.9 , salts have pH of 7 and bases have pH ranging from 7.1 to 14.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2022/formulas/chemistry/college/jqpk2yoscrvrwkn7a8mwhb95o3yruvgdhz.png)
Putting in the values:
![pH=-\log[5.6* 10^(-9)]](https://img.qammunity.org/2022/formulas/chemistry/college/7bvwu5iifdyjskcmqnl9s5v5fdqaa217qp.png)
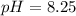
Thus as pH is more than 7, the solution is basic.
The pH of the substance is 8.25. The solution is basic.