Answer:
7.97 moles of neon are present in the canister.
Step-by-step explanation:
Avogadro's constant or "Avogadro's number" is the number of constituent particles found in the amount of substance in one mole.
In other words, Avogadro's number is the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023*10²³ particles per mole. Avogadro's number applies to any substance.
So, you can apply the following rule of three: if 6.023*10²³ atoms are present in 1 mole, 4.8*10²⁴ atoms are present in how many moles?
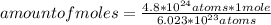
amount of moles= 7.97 moles
7.97 moles of neon are present in the canister.