Answer:
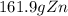
Step-by-step explanation:
Hello there!
In this case, according the given balanced chemical reaction:
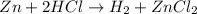
It is possible to evidence the 1:1 mole ratio of hydrogen (molar mass = 2.02 g/mol) to zinc (atomic mass = 65.41 g/mol) which is used to calculate the grams of the latter needed for the production of 5 grams of the former via stoichiometry:
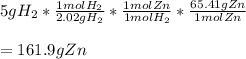
Best regards!