Answer:
The concentration of this NaCl solution is 0.0769M
Step-by-step explanation:
Hello there!
In this case, since we are dealing with a solubility equilibrium in which it is desired to compute the concentration of a common-ion effect to chloride solution in the form of NaCl, we can set up the equilibrium reaction and expression:
![AgCl(s)\rightleftharpoons Ag^+(aq)+Cl^-(aq)\\\\Ksp=[Ag^+][Cl^-]\\](https://img.qammunity.org/2022/formulas/chemistry/high-school/ogs0gkc5wlxv31ooliiw362xv6otfikj83.png)
It is possible to insert the concentration of starting chloride ions provided by the ionization of NaCl and in terms of the molar solubility, s, equal to 2.38x10^{-9}M:
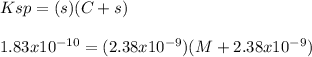
Thus, solving for M, we obtain:
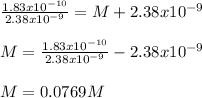
Thus, the concentration of this NaCl solution is 0.0769M.
Best regards!