Answer:
The average kinetic energy is increased by a factor of 3.
Step-by-step explanation:
The average kinetic energy is given by:
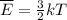
Where:
k: is the Boltzmann constant
T: is the temperature
When the temperature is 300 K we have:
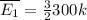
Now, when the temperature is 900 K:
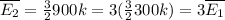
Therefore, when the temperature is raised from 300 K to 900 Κ, the average kinetic energy is increased by a factor of 3.
I hope it helps you!