Answer:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
190.14 mL of the NaOH solution would be required.
Step-by-step explanation:
The balanced reaction is:
- 2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
Now we calculate how many H₂SO₄ moles are there in 25.00 mL of a 5.400 M solution, using the definition of molarity:
- Molarity = moles / liters
- Moles = Molarity * liters
- 25.00 mL * 5.400 M = 135 mmol H₂SO₄
Now we convert H₂SO₄ moles into NaOH moles, using the stoichiometric coefficients of the balanced reaction:
- 135 mmol H₂SO₄ *
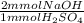 = 270 mmol NaOH
= 270 mmol NaOH
Finally we calculate the volume of a 1.420 M solution that would contain 270 mmoles:
- Molarity = moles / liters
- liters = moles / molarity
- 270 mmol / 1.420 M = 190.14 mL