Answer:
Approximately
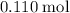 .
.
Step-by-step explanation:
Balanced equation for this reaction:
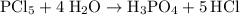 .
.
Look up the relative atomic mass of
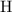 and
and
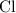 on a modern periodic table:
on a modern periodic table:
Calculate the molar mass of
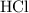 :
:
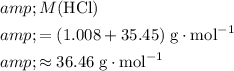 .
.
Calculate the number of moles of molecules in that
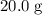 of
of
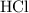 :
:
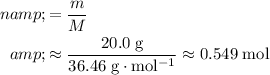 .
.
In the balanced reaction, the coefficient of
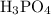 and
and
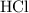 are
are
 and
and
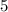 , respectively. The ratio between these two coefficients is:
, respectively. The ratio between these two coefficients is:
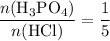 .
.
In other words, this reaction would produce five times as many
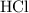 molecules as
molecules as
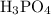 molecules.
molecules.
Calculation shows that in this question, approximately
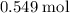 of
of
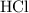 molecules were produced. Calculate the number of moles of
molecules were produced. Calculate the number of moles of
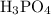 molecules that were produced:
molecules that were produced:
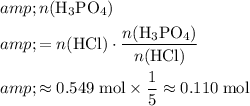 .
.