Answer: The rate increases 3 times on raising the temperature from 20degree to 30 degree
Step-by-step explanation:
According to Arrhenius equation with change in temperature, the formula is as follows.
![ln (k_(2))/(k_(1)) = (-E_(a))/(R)[(1)/(T_(2)) - (1)/(T_(1))]](https://img.qammunity.org/2022/formulas/chemistry/college/y95oqc16xhfspirxcw3imk40ehtx0adjz2.png)
where
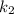 = rate constant at temp
= rate constant at temp
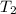
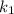 = rate constant at temp
= rate constant at temp
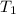
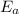 = activation energy
= activation energy
R= gas constant
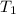 = temperature =
= temperature =
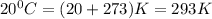
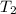 = temperature =
= temperature =
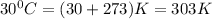
![ln (k_(2))/(k_(1)) = (-85* 1000J/mol)/(8.314J/Kmol)[(1)/(303) - (1)/(293)]](https://img.qammunity.org/2022/formulas/chemistry/college/2z1iwmgo7qjvwf9eh4nsuqokc2qfez5dmq.png)
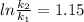
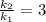
Thus rate increases 3 times on raising the temperature from 20degree to 30 degree