Answer:
0.60 moles
Step-by-step explanation:
From the reaction:
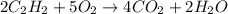
From above, Only 2 moles of CH2CH2 are required to produce 2 moles of water (H_2O).
As such, 0.6 moles of H2O will require:
x (CH2CH2) × 2 moles of (H_2O) = 0.6 moles (CH2CH2) × 2 mole of (H_2O)
x mole of (CH2CH2) = 0.60 moles
∴
0.6 moles of H2O will require 0.60 moles of CH2CH2