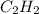Answer: 6.38 moles of
 are needed to react with 2.55 mol of
are needed to react with 2.55 mol of
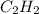
Step-by-step explanation:
The balanced chemical reaction for the combustion of ethyne is:
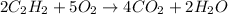
According to stoichiometry:
2 moles of
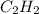 require = 5 moles of
require = 5 moles of

Thus 2.55 mol of
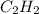 require =
require =
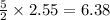 moles of
moles of

Thus 6.38 moles of
 are needed to react with 2.55 mol of
are needed to react with 2.55 mol of