Answer:
1.07 g Ba
Step-by-step explanation:
Hello there!
In this case, according to the definition of the Avogadro's number and the molar mass, it is possible to say that 6.022x10^{23} atoms of barium equal one mole, and at the same time, 1 mole equals 137.327 grams of this element; thus, it is possible to say that 6.022x10^{23} atoms of barium have a mass of 137.327 grams; therefore, it i possible for us to calculate the required mass in grams as shown below:
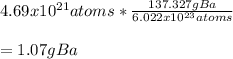
Best regards!