Solution :
Assuming all Al will be consumed in the reaction.
From the given equation, 2 mole of Al produce 2 mole of
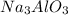 .
.
So, 1 mole of Al produce 1 mole of
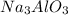 . .....statement 1)
. .....statement 1)
Number of moles of Al in 258 gram of Al.
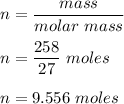
Therefore, from statement 1) number of moles of
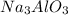 produced is also 9.556 moles.
produced is also 9.556 moles.