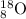Answer: The complete nuclear symbol will be
Step-by-step explanation:
The nuclear symbol is written as:
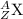 where Z= atomic number , A = mass number X = symbol of element
where Z= atomic number , A = mass number X = symbol of element
Atomic number is defined as the number of protons or number of electrons that are present in an atom.
For an electrically neutral atom,
Atomic number = Number of electrons = Number of protons = 8
Mass number is defined as the sum of number of protons and neutrons that are present in an atom.
Mass number = Number of protons + Number of neutrons
Mass number = 8 + 10 = 18
Thus complete symbol will be