Answer:
pH = 2.88
Step-by-step explanation:
For a weak acid solution, the pH can be calculated using the following formulas:
- [H⁺] =
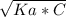
Where Ka =
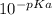 and C is the molar concentration.
and C is the molar concentration.
We are given Ka and C by the problem, meaning we can now proceed to calculate [H⁺]:
- [H⁺] =
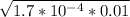
Finally we calculate the pH:
- pH = - log (0.0013) = 2.88