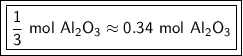
Answer:
Step-by-step explanation:
We will use stoichiometry to solve this problem. The reaction given has a formula of
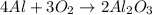
The coefficients tell us the number of moles necessary for the reaction.
The reaction requires 3 moles of oxygen to produce 2 moles of aluminum oxide. We can make a ratio.
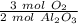
Since we are doing the reaction with 0.5 moles of oxygen, we multiply the ratio by that number.
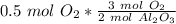
Flip the ratio so the moles of oxygen cancel each other out.
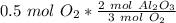
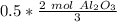
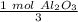
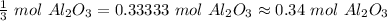
0.5 moles of oxygen produces 1/3 or approximately 0.34 moles of aluminum oxide.