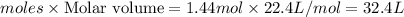Answer: 1. 20.0 grams
2. 0.272 moles
3. B) Mass --> Moles --> Volume
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance weighs equal to molecular mass and contains avogadro's number
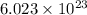 of particles.
of particles.
To calculate the number of moles, we use the equation:
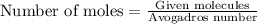 or
or
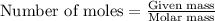 or
or
Putting in the values we get:
1.
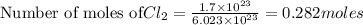
Mass of
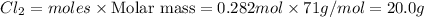
2.
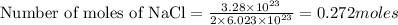
3.
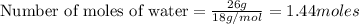
Volume of water =