Answer:
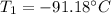
Step-by-step explanation:
Hello there!
In this case, given the T-V variation, we understand it is possible to apply the Charles' law as shown below:
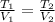
Thus, since we are interested in the initial temperature, we can solve for T1, plug in the volumes and use T2 in kelvins:

Best regards!