Given :
Temperature, T = 55° C = ( 55 + 273 ) K = 328 K .
Volume of container, V = 28.9 dm³ = 0.0289 m³ .
Pressure, P = 144 kPa = 144000 Pa .
To Find :
Number of moles of nitrogen gas.
Solution :
We know, by ideal gas equation :
PV = nRT ( R ( universal gas constant ) = 8.31 J K⁻¹ mol⁻¹ .
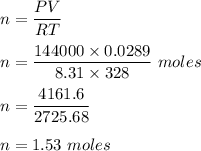
Therefore, number of moles of nitrogen gas is 1.53 moles.