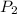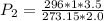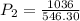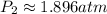Question:
At standard temperature and pressure, the volume of a tire is 3.5L. What is the new pressure if the temperature outside is 296k and its weight causes the volume of the gas is 2.0 L?
Answer:
The new pressure is: 1.896 atm
Step-by-step explanation:
At standard temperature and pressure, we have:
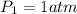
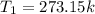
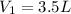
Outside, we have:
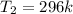
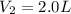
Required
Determine the new pressure
Using combined gas law, we have:
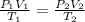
This gives:
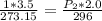
Solve for