Sum of ionisation energies is very high.
Let's check once .
Group 13 means boron family.
- Take example of Aluminium (Al)
- Atomic no 13
Electronic configuration is given by
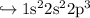
Look at p orbital .
- It's half filled.
- So it's far stable than other group's elements except noble gases
Its first ionisation enthalpy is that much high that it needs a lots of energy to form ionic compounds by donating that.
- Second and third enthalpies becomes more and more high as it closes the nucleus.
So boron family is unable to form ionic compounds easily .
- So they prefer sharing electrons and forming covalent compounds