Answer:
![[N_2]=0.0866M](https://img.qammunity.org/2022/formulas/chemistry/college/89jcb6fupnt3zysfkt38x1t7oev8827id0.png)
Step-by-step explanation:
Hello there!
In this case, in agreement to the chemical reaction, it is possible for us to figure out the equilibrium concentration of the N2 product, via an ICE table plugged in the equilibrium expression:
![Kc=([N_2][O_2])/([NO]^2)\\\\2.4x10^3=(x*x)/((0.175-2x)^2)](https://img.qammunity.org/2022/formulas/chemistry/college/4rhrwx368tugolgiyh5fktvaj4fpvk2ny9.png)
In such a way, when solving for x via quadratic equation or just a solver, it is possible to obtain:
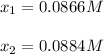
In such a way, since the root 0.0884 M produce a negative concentration of NO (0.175-2*0.0884=-0.0018M), we infer that the correct root is 0.0866 M; therefore, the concentration of N2 at equilibrium is equal to x:
![[N_2]=x=0.0866M](https://img.qammunity.org/2022/formulas/chemistry/college/1rgbrj478bgdsx05mjbq4783zwgaf8vp34.png)
Best regards!