Answer:
m = 176.04 g .
Step-by-step explanation:
Hello there!
In this case, according to the mole-mass relationships, which are based off the mass of one mole of any compound via the molar mass, it is possible to realize that the molar mass of carbon dioxide is 44.01 (12.01+16*2) g/mol, and therefore, the mass in grams of 4.00 moles of this compound are calculated as shown below:
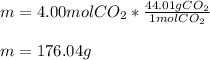
Best regards!