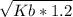Answer:
Kb = 3.64x10⁻⁵
Step-by-step explanation:
First we calculate the pOH of the solution:
Then we calculate [OH⁻]:
- [OH⁻] =
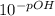
For a weak base solution with a relatively high concentration, we can use the following formula:
- [OH⁻] =
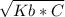
Where C is the concentration of the weak base solution (1.2 M in this case).
With the formula above we can calculate Kb:
- 0.0066 =