Answer: The rate will increase 32 times if the concentration of both reactants increases 4 times
Step-by-step explanation:
Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
For the reaction :
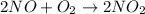
![rate=k[NO]^2[O_2]^1](https://img.qammunity.org/2022/formulas/chemistry/high-school/32xv6n2hzumdp1n9r7so8yqbatbizoxo2o.png)
k= rate constant
order with respect to
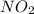 = 2
= 2
order with respect to
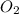 = 1
= 1
Thus on increasing the concentration of reactants 4 times :
![rate'=k[4* NO]^2[4* O_2]^1](https://img.qammunity.org/2022/formulas/chemistry/high-school/owm3b36e5efq5n9aya0zgnqlh9zz4e00rr.png)
![rate'=k* 8* 4* [NO]^2* [O_2]^1](https://img.qammunity.org/2022/formulas/chemistry/high-school/axddefmvicro0daotu84cevk3zv6hb1hal.png)
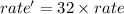
Thus rate will increase 32 times if the concentration of both reactants increases 4 times