Answer:
The value of the equilibrium constant Kc is 5.45
Step-by-step explanation:
The "Law of Mass Action" states:
"For a reversible reaction in chemical equilibrium at a given temperature, it is true that the product of the concentrations of the products raised to the stoichiometric coefficients divided by the product of the concentrations of the reactants raised to their stoichiometric coefficients is a constant."
This constant was called the equilibrium constant. For a reaction:
aA + bB ⇄ cC + dD
the equilibrium constant Kc is:
![Kc=([C]^(c) *[D]^(d) )/([A]^(a) *[B]^(b) )](https://img.qammunity.org/2022/formulas/chemistry/high-school/bqtwr85tvvfqy5lkvl057xxprvr50i38mv.png)
In this case, the balanced reaction is:
2 H₂S → 2 H₂ + S₂
So, the equilibrium constant Kc is:
![Kc=([H_(2) ]^(2) *[S_(2) ] )/([H_(2) S]^(2) )](https://img.qammunity.org/2022/formulas/chemistry/high-school/ghnnzwrghfzq5u4kke8yq4cz81y03a6mau.png)
The equilibrium concentrations are
- [H₂S] =0.25 M
- [H₂]= 0.88 M
- [S₂]= 0.44M
Replacing in the definition of equilibrium constant:
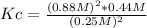
Solving:
Kc= 5.45
The value of the equilibrium constant Kc is 5.45