Answer:
46.76 g
Step-by-step explanation:
The reaction that takes place is:
First we determine the limiting reactant:
1.3 moles of SiO₂ would react completely with (3 * 1.3) 3.9 moles of C. There are not as many C moles, meaning that C is the limiting reactant.
Now we convert C moles (the limiting reactant) into CO moles, using the stoichiometric coefficients:
- 2.5 mol C *
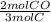 = 1.67 mol CO
= 1.67 mol CO
Finally we convert 1.67 moles of CO to grams, using its molar mass:
- 1.67 mol CO * 28 g/mol = 46.76 g