Answer: The volume of diluted solution is 4 L
Step-by-step explanation:
According to the dilution law,
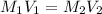
where,
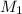 = molarity of stock
= molarity of stock
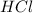 solution = 8 M
solution = 8 M
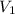 = volume of stock
= volume of stock
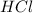 solution = 1 L
solution = 1 L
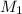 = molarity of diluted
= molarity of diluted
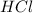 solution = 2 M
solution = 2 M
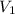 = volume of diluted
= volume of diluted
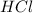 solution = ?
solution = ?
Putting in the values we get:
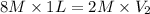
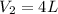
Therefore, volume of diluted solution is 4 L