Answer:
Moving left to right across each row from top to bottom:
(skipping filled spaces)
15, 13, 10, 13, 28
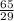 Cu, 36, 29
Cu, 36, 29
122, 82, 82, 82, 204
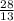
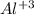 , 15, 13
, 15, 13
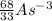 , 33, 68
, 33, 68
How to do it: Each symbol represents an element. Each element has its mass # above its atomic # next to its abbreviated symbol. The # of neutrons is the mass # - the atomic #. The # of protons is the same as the atomic #. It gets tricky with the electrons, so pay attention. Neutrons have no charge, so forget about them for now. Protons are positively charged, and electrons are negatively charged. If you look at some of those symbols, the Al (aluminum) has a number above it, to its right. That is its charge. The charge is usually zero, and isn't included, unless the # of protons and # of electrons are different. If so, subtract the # of electrons from the # of protons (protons - electrons = charge), which will give you the charge. Be sure to include the +/- sign when writing the charge. The # of protons + the # of neutrons is the mass #.
Leave a comment on my answer if there is anything you need me to explain in more detail.