Answer: The molarity of solution is 0.08 M
Step-by-step explanation:
pH is the negative logarithm of hydrogen ion concentration. It tells abpout the acidity or basicity of a solution.
![pH=-log[H^+]](https://img.qammunity.org/2022/formulas/chemistry/high-school/9mqx86gdr4r9esyzqxbjxb9ugmy542eyxn.png)
also pH+pOH=14
given : pH of NaOH = 12.9
Thus pOH = (14-12.9) = 1.1
Thus
![[OH^-]=10^(-1.1)=0.08M](https://img.qammunity.org/2022/formulas/chemistry/high-school/6qb6zzo4k5yrukw1fxrl52zus9mcml8bh6.png)
As
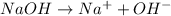
As 1 mole of
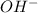 is produced by = 1 mole of NaOH
is produced by = 1 mole of NaOH
Thus 0.08 moles of
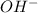 are produced by =
are produced by =
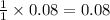 moles of NaOH
moles of NaOH
The molarity of solution is 0.08 M