Answer:
C. 30 kJ
Step-by-step explanation:
Hello there!
In this case, in agreement to the thermodynamic definition of the Gibbs free energy, in terms of enthalpy of entropy:
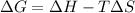
It is possible to calculate the required G by plugging in the given entropy and enthalpy as shown below:
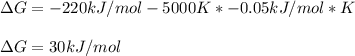
Therefore, the answer is C. 30 kJ .
Best regards!