Answer:
m = 998 g
Step-by-step explanation:
Hello there!
In this case, according to the definition of the molar mass as the mass of one mole of the compound, it is possible to state the 1 mole of C8H18 has a mass of 114.26 grams; therefore, the mass in 8.65 moles turn out to be:
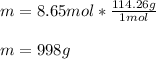
In agreement to the notation requirement.
Best regards!