Answer:
Q = 114349.5 J
Step-by-step explanation:
Hello there!
In this case, since this a problem in which we need to calculate the total heat of the described process, it turns out convenient to calculate it in three steps; the first one, associated to the heating of the liquid water from 40 °C to 100 °C, next the vaporization of liquid water to steam at constant 100 °C and finally the heating of steam from 100 °C to 115 °C. In such a way, we calculate each heat as shown below:
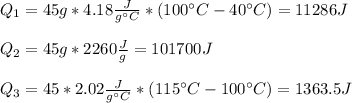
Thus, the total energy turns out to be:
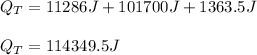
Best regards!