Answer:
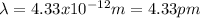
Step-by-step explanation:
Hello there!
In this case, since the speed, wavelength and mass of an electron are related via the the Broglie wavelength:
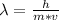
Thus, by plugging in the mass of the electron and the Planck's constant, we obtain the following wavelength:
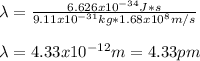
Best regards!