Answer:
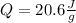
Step-by-step explanation:
Hello there!
In this case, according to the equation for the calculation of the heat during a heating process:
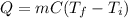
It is possible to compute it per gram of ice by just removing m from the equation by dividing at both sides. Next we plug in the given specific heat and the final and initial temperatures to obtain:
![Q=2.06(J)/(g\°C)[-15\°C-(-25\°C)] \\\\Q=20.6(J)/(g)](https://img.qammunity.org/2022/formulas/chemistry/high-school/vhqfq1jn436c0p18va3k7nc7dnol1v114o.png)
Best regards!