Answer:
Approximately
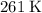 , if this gas is an ideal gas, and that the quantity of this gas stayed constant during these changes.
, if this gas is an ideal gas, and that the quantity of this gas stayed constant during these changes.
Step-by-step explanation:
Let
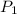 and
and
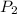 denote the pressure of this gas before and after the changes.
denote the pressure of this gas before and after the changes.
Let
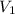 and
and
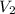 denote the volume of this gas before and after the changes.
denote the volume of this gas before and after the changes.
Let
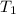 and
and
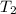 denote the temperature (in degrees Kelvins) of this gas before and after the changes.
denote the temperature (in degrees Kelvins) of this gas before and after the changes.
Let
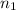 and
and
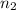 denote the quantity (number of moles of gas particles) in this gas before and after the changes.
denote the quantity (number of moles of gas particles) in this gas before and after the changes.
Assume that this gas is an ideal gas. By the ideal gas law, the ratios
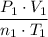 and
and
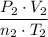 should both be equal to the ideal gas constant,
should both be equal to the ideal gas constant,
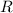 .
.
In other words:
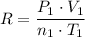 .
.
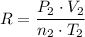 .
.
Combine the two equations (equate the right-hand side) to obtain:
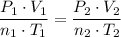 .
.
Rearrange this equation for an expression for
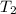 , the temperature of this gas after the changes:
, the temperature of this gas after the changes:
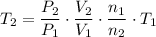 .
.
Assume that the container of this gas was sealed, such that the quantity of this gas stayed the same during these changes. Hence:
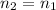 ,
,
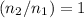 .
.
![\begin{aligned} T_2 &= (P_2)/(P_1) \cdot (V_2)/(V_1) \cdot (n_1)/(n_2)\cdot T_1 \\[0.5em] &= (1.67\; \rm atm)/(1.12\; \rm atm) * (11.2\; \rm m^(3))/(15.7\; \rm m^(3)) * 1 * 245\; \rm K \\[0.5em] &\approx 261\; \rm K\end{aligned}](https://img.qammunity.org/2022/formulas/physics/college/59uvlq1z3jznsm5eajt61v0enwvs5bb806.png) .
.