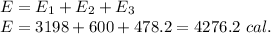Answer: 4276.2 calories
Step-by-step explanation:
Given
mass of steam is 6 gm at
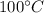
Conversion of steam to ice involves
- steam to water at
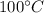
- water at
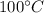 to water at
to water at
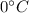
- water to the ice at
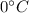
Calories released during the conversion of steam to water at
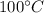
![E_1=mL_v\quad [L_v=\text{latent heat of vaporisation}]\\E_1=6* 533=3198\ cal.](https://img.qammunity.org/2022/formulas/physics/high-school/oc3gxyzat3gj9qf4x21ny7d1xdu0num5ek.png)
Calories released during the conversion of water at
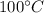 to water at
to water at
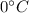
![E_2=mc(\Delta T)\quad [c=\text{specific heat of water,}1\ cal./gm.^(\circ)C]\\E_2=6* 1* 100=600\ cal.](https://img.qammunity.org/2022/formulas/physics/high-school/2juszkvgmm940n1ke98s8yss7acbveyvzk.png)
Calories released during the conversion of water to the ice at
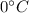
![E_3=mL_f\quad [L_f=\text{latent heat of fusion}]\\E_3=6* 79.7=478.2\ cal.](https://img.qammunity.org/2022/formulas/physics/high-school/xu6unu4a50v1bp2rttje4e4wx2dpt5eip5.png)
The total energy released is