Answer:
The mass of the mercury remaining in the bottle is 497.57 grams.
Step-by-step explanation:
The mass of the mercury remaining in the bottle is found by subtracting the mass expeled due to heating from initial mass inside the bottle. That is:
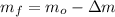 (1)
(1)
Where:
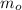 - Initial mass, in grams.
- Initial mass, in grams.
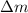 - Mass expelled due to heating, in grams.
- Mass expelled due to heating, in grams.
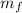 - Final mass, in grams.
- Final mass, in grams.
If we know that
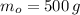 and
and
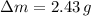 , then the mass of the mercury remaining in the bottle is:
, then the mass of the mercury remaining in the bottle is:
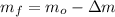
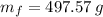
The mass of the mercury remaining in the bottle is 497.57 grams.