Answer:
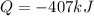
Step-by-step explanation:
Hello there!
In this case, considering that the heat has two forms, sensible (variable temperature) and latent (constant temperature), we can notice that phase changes account for latent heat as the temperature remains the same. In such a way, given the enthalpy of vaporization of water, 40.67 kJ/mol, the enthalpy of condensation (reverse process) is the negative value, -40.67 kJ/mol; therefore, the associated latent heat would be:
Best regards!