Answer:
A. your running speed 1.5 m/s
B. your mass 70 kg
C. your de Broglie wavelength
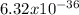 m
m
Step-by-step explanation:
Hello there!
In this case, since the equation for the calculation of the Broglie wavelength is:
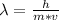
We can assume a running speed of about 1.5 m/s and a mass of 70 kg, so the resulting Broglie wavelength is:
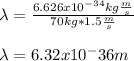
Best regards!