Answer:
![[Ag^+]=1.3x10^(-5)M](https://img.qammunity.org/2022/formulas/chemistry/college/unmdvcx22jdlf8c40x0svvfrcnvyq9r5ac.png)
Step-by-step explanation:
Hello there!
In this case, according to the dissociation of silver chloride in aqueous solution, we can write:
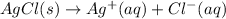
Whereas the equilibrium expression is:
![Ksp=[Ag^+][Cl^-]](https://img.qammunity.org/2022/formulas/chemistry/college/258g5rj7wu5xs4rwcazox93rxl9nqg1bpv.png)
And the solubility product constant is 1.7 x10⁻¹⁰; thus, by inserting x as the concentration of both silver and chloride ions as they are the same at equilibrium, we obtain:
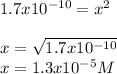
Which is also equal to the concentration silver ions at equilibrium in a saturated solution because we considered the Ksp.
![[Ag^+]=1.3x10^(-5)M](https://img.qammunity.org/2022/formulas/chemistry/college/unmdvcx22jdlf8c40x0svvfrcnvyq9r5ac.png)
Best regards!