Answer:
The answer is "Option b and Option c".
Step-by-step explanation:
This buffer is a buffer of ammonia and ammonium ion. Thus it requires the solution
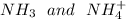 .
.
In point 1:
The solution containing
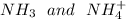 at 1M concentration would be given by mixing the two solutions. Thus, this buffer is a legitimate route.
at 1M concentration would be given by mixing the two solutions. Thus, this buffer is a legitimate route.
In point 2:
It gives the ions you want but they are not the same.
In point 3:
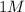
 and
and
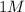
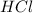 volume would not produce the same
volume would not produce the same
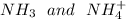 concentrations. Therefore, this buffer isn't a valid route.
concentrations. Therefore, this buffer isn't a valid route.
In point 4:
Some
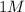
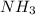 volume and half
volume and half
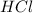 . This offers the same rate as half.
. This offers the same rate as half.