Answer:
E 3.0 x 10² mL.
Step-by-step explanation:
Hello there!
In this case, according to the formula for the calculation of the mass-volume percent:
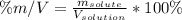
Whereas it is necessary to know the mass of the solute and the volume of the solution. Thus, given the mass of NaOH as the solute, the volume of the solution would be:
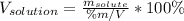
Then, by plugging in we obtain:
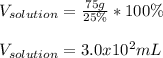
Thus, the answer is E 3.0 x 10² mL.
Best regards!