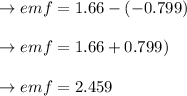Answer:
The answer is "2.459".
Step-by-step explanation:
The cell potential or emf norm = oxidation pot. of anode -oxidation pot. of cathode
When the pot oxidizer is the opposite of a red pot size it's been transformed to either the redox reaction so that anode was its higher oxide pot material that means (AL) so,