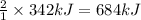Answer:
Step-by-step explanation:
The skeletal thermochemical equation for the reaction is:
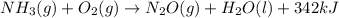
The balanced thermochemical equation for the reaction is:
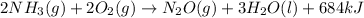
When 1 mole of
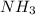 reacts with oxygen , heat released = 342 kJ
reacts with oxygen , heat released = 342 kJ
Thus when 2 moles of
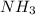 reacts with oxygen , heat released =
reacts with oxygen , heat released =