The given question is incomplete. The complete question is:
Gallium (Ga, 69.723 g/mol) is a metalloid obtained from its salts during the smelting of ores of other elements, like Zinc. has broad applicability in the electronics industry. It is also used as a safe replacement for mercury in thermometers as it melts at 29.8 °C and has a heat of fusion of 5.59 kJ/mol. What is the entropy change of 22 g of gallium in J/K as it melts when placed on a surface at 29.8°C?
Answer: The entropy change of of gallium in as it melts when placed on a surface at is 5.81 J/K
Step-by-step explanation:
Latent heat of fusion is the amount of heat required to convert 1 mole of solid to liquid at atmospheric pressure.
Amount of heat required to melt 1 mole of Ga = 5.59 kJ
Mass of Ga given = 22 gram
Heat required to melt 69.723 g of Ga = 5.59 kJ
Thus Heat required to melt 22 g of Ga =
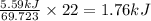
Temperature =
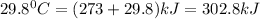
Now entropy change =
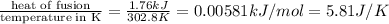
Thus the entropy change of of gallium in as it melts when placed on a surface at
 is 5.81 J/K
is 5.81 J/K